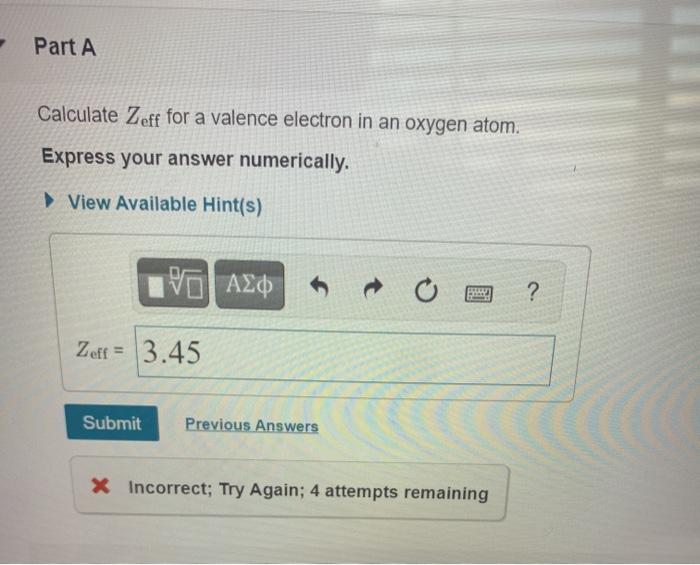
23+ Calculate Zeff For A Valence Electron In An Oxygen Atom.
There are 15 atoms in the first model 11. 19 - 15 4 so the other models oxygen is the fifth atom in the file Align two structures on top of each other using.

How To Calculate Effective Nuclear Charge Sciencing
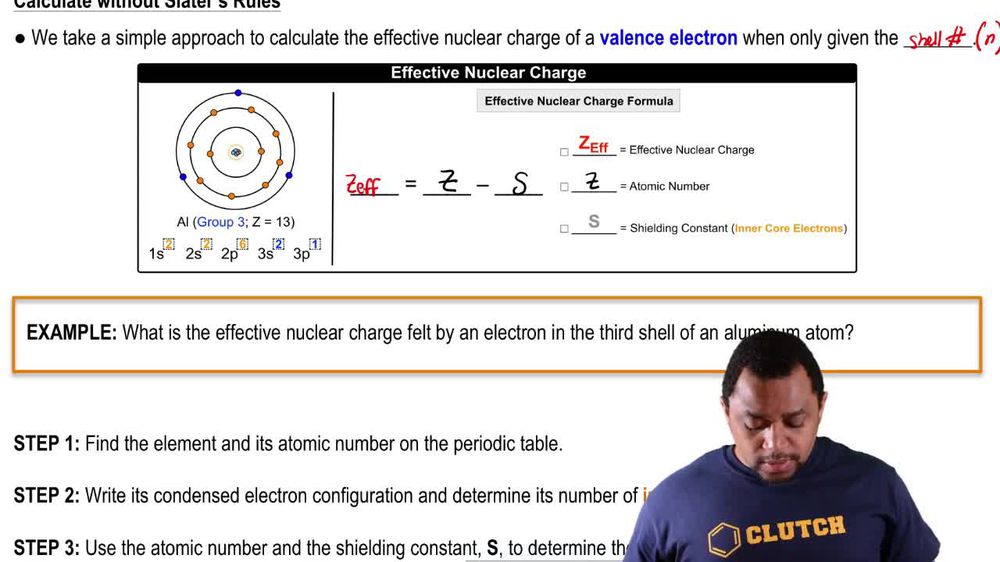
8 O 1s2 2s2 2p4.

. TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc. A number of polyatomic anions containing oxygen atoms are named based on the root word of the central or non-oxygen atom and the suffix ate for the one with more oxygen atoms and ite for the one with less oxygen atom. Titration is based on a reaction between the analyte unknown sample and the regent of known concentration and reaction stoichiometry.
14 mol C H 12011 g 10079 g 14007 g 18 mol H 2 mol N mol C mol H mol N 5 mol O b. For example in period 4 element 23 vanadium has an electron configuration of Ar3d34s2 but element 24 chromium has an electron configuration of Ar. How do I calculate preparation of mobile phase 25mM ammonium acetate in 01L from ammonia 25 and.
TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc. The valence electrons in an oxygen atom box are attracted to the nucleus by a positive charge nearly double 1s2-1box2s2-1box2p3-3boxes that of boron. Nuclear charge Zeff experienced by the electron present in them.
How is the effective nuclear charge. It is the net positive charge experienced by an electron in a question_answer Q. Enter the email address you signed up with and well email you a reset link.
In this case m3 the oxygen atom is 0 19. S orbitals shield the electrons from the nucleus more than p-orbitals which shield more in d. In two chemical reactions 40 grams of oxygen combine with 30 grams of carbon to form one compound and 80 grams of oxygen combine with 3 grams of carbon to form another.
A parameter which characterises any atom or ion is the so-called effective The element sodium has the electron configuration 1s 2 2s 2 2p 6 3s 1. In a multi-electron atom which orbital will have the highest energy. The closer an atom is to having a half-full valence shell the higher the melting point high.
That is the oxygen is the first atom in the the first model from PubChem and the 5th atom in the second model from NCICADD. Similarly when an atom gains electrons the resulting anion is larger owing to both increased electron-electron repulsions and a. 15999 g 294305 gmol mol O 1 mol C14 H18 N 2 O 5 340 102 mol C14H18N2O5 2943 g C14 H18 N 2 O 5 2943 g 459 g C14H18N2O5 mol 1g 1 mol 602 10.
100 g C14H18N2O5 c. The ion with the electron configuration of 1s²2s²2p⁶3s²3p⁶3d³ has a total of 21 electrons. Titration is based on a reaction between the analyte unknown sample and the regent of known concentration and reaction stoichiometry.
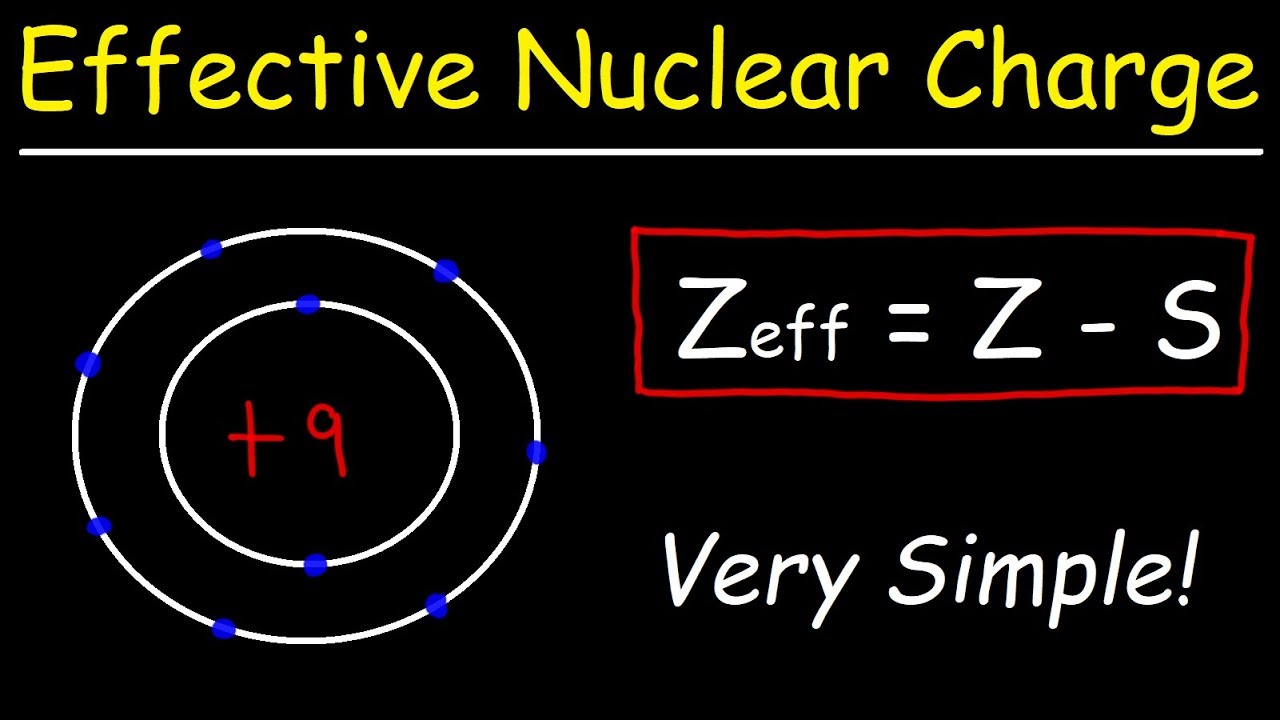
The effective nuclear charge Zeff. SO42- sulfate ion e. The symbol 133Cs133Cs indicates that the mass number is 133.
Greater than When an electron in excited energy level drops to a lower energy level a photon is emitted. The present paper describes a new tripodal ligand containing imidazole and pyridine arms and its first cis-RuIIILCl2ClO4 complex 1. Calculate the number of neutrons from the mass number and the number of protons.
133 55 x 78 x. 31. 50 mg e.
NO2- nitrite ion c. Calculate ΔHrxn for the reduction for V2O5. 156 mol d.
Transcript 10 mol F2 440 g CO2 40 g H2 146 g SF6 34. The crystal structure of 1 shows RuIII in a distorted octahedral geometry in which two chloride ions cis-positioned to each other are coordinated besides the four nitrogen atoms from the tetradentate ligand L. Question 13 2 pts Calculate the effective nuclear charge experienced by a beryllium valence electron.
Nickel atom can lose two electrons to. Cesium has an atomic number of 55 indicating it has 55 protons and 55 electrons in the neutral atom. Therefore 7 m 3 would equal 7 10 3 L which is close to the answer.
Enter the email address you signed up with and well email you a reset link. In the Bohr model of the hydrogen atom the energy required to excite an electron from n 2 to n 3 is _____ the energy required to excite an electron from n 3 to n 4. The attraction between this lone valence electron and the nucleus with 11 protons is shielded by the other 10 core electrons.
The mass number of an atom is representative of the number of protons and neutrons. Of the options given the only ion with the same number of electrons is V² resulting from the electron configuration of 1s²2s²2p⁶3s²3p⁶4s²3d³ 23 total electrons losing two electrons from the. It is important to keep in mind that this filling is not always regular.
In period 4 of the table the 3d subshell fills and in periods 5 and 6 the 4d and 5d subshells fill respectively. From the above conversion factors you can show that 1 m 3 1 10 3 L. SO32- sulfite ion d.
Show the distribution of electrons in oxygen atom atomic number 8 using. NO3- nitrate ion b. O Zet 100 o Zeff 250 o Zeff 124 O Zeff - 075 o Zeff - 195 D Question 14 2 pts Select the valence electron configuration of a group 2 elementSolutions for Chapter 7 Problem 11Q.
The outer energy level is n 3 and there is one valence electron.

Solved Part A Calculate Zeff For A Valence Electron In An Oxygen Atom Express Your Answer Numerically View Available Hint S Azd Zeff 3 45 Submit Previous Answers Incorrect Try Again 4 Attempts Remaining
Periodic Trend Effective Nuclear Charge Video Tutorial Practice Pearson Channels
How To Find The Effective Nuclear Charge Of Oxygen Quora

Pdf Electron Impact Excitation Cross Section Measurement For N 3 To N 2 Line Emission In Fe17 To Fe23

Ions Effective Nuclear Charge Of Oxygen Atom O Vs Oxygen Anion O2 Chemistry Stack Exchange
How To Calculate The Effective Nuclear Charge Of An Electron Youtube

Periodic Trends Wikipedia
Solved Part A Calculate Zeff For A Valence Electron In An Chegg Com

Computational Chemis

10th Science Em Pdf Science Notes Teachmint
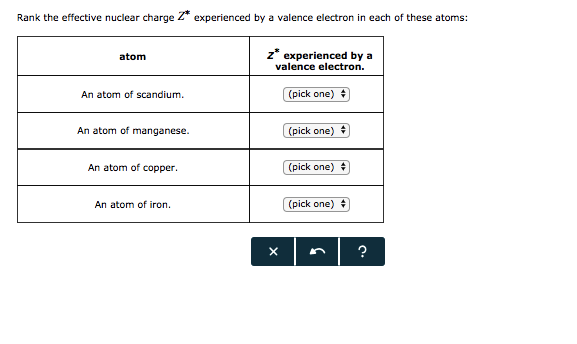
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com

Relativistic Heavy Neighbor Atom Effects On Nmr Shifts Concepts And Trends Across The Periodic Table Chemical Reviews

Relativistic Heavy Neighbor Atom Effects On Nmr Shifts Concepts And Trends Across The Periodic Table Chemical Reviews

Pdf Electron Impact Excitation Cross Section Measurement For N 3 To N 2 Line Emission In Fe17 To Fe23
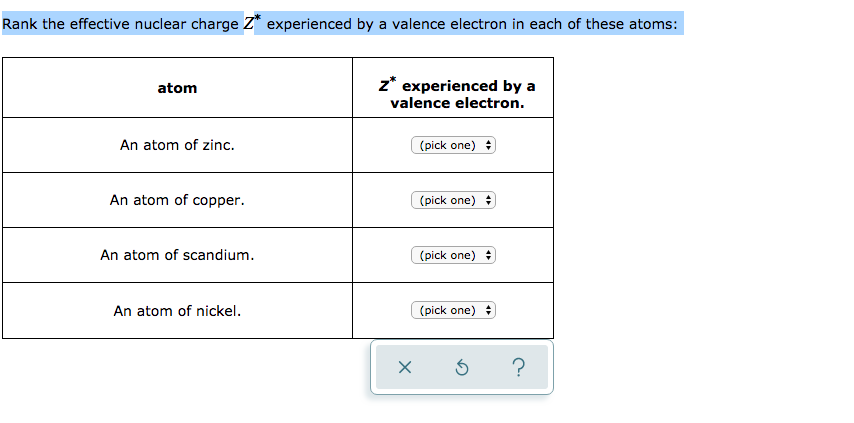
Answered Rank The Effective Nuclear Charge Z Bartleby

Pdf Spectroscopic Study Of Fe 2 O 2 5 Et 3 Tpa 2 3 Nature Of The Fe 2 O 2 Diamond Core And Its Possible Relevance To High Valent Binuclear Non Heme Enzyme Intermediates Lawrence Que Academia Edu
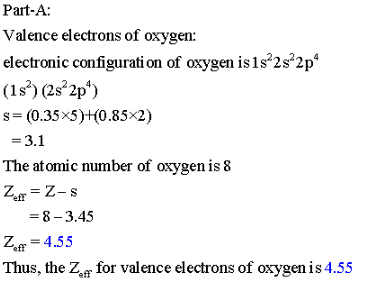
Part A Calculate Zeff For A Valence Electron In An Oxygen Atom Home Work Help Learn Cbse Forum